Abstract
Background
Early T-precursor acute lymphoblastic leukemia (ETP-ALL) is a subclass of T-cell ALL (T-ALL) accounting for about 15% of pediatric T-ALL cases and characterized by an immature T cell phenotype, resistance to therapy, and high rates of induction failure and relapse (Wood B et al Blood 2009). MERTK receptor tyrosine kinase is not expressed in normal T cells but is ectopically expressed in 40-50% of T-ALLs, particularly those with an immature T cell phenotype (Graham DK et al Clin Cancer Res 2006), suggesting a role in ETP-ALL. Inhibition of MERTK using shRNA delayed leukemia progression and prolonged survival in a T-ALL xenograft model, implicating MERTK as a therapeutic target (Brandao LN et al Blood Cancer J 2013). Fms-like tyrosine kinase 3 (FLT3) mRNA is overexpressed in ETP-ALL relative to typical T-ALL (Kawashima-Goto S et al PLoS One 2015) and activating FLT3 mutations are present in 14-35% of ETP-ALLs (Zhang J et al Nature 2012; Neumann M et al PLoS One 2013); MRX-2843 is a novel, orally-bioavailable, dual MERTK and FLT3 tyrosine kinase inhibitor developed by our group that was recently granted IND approval from the FDA and thus, MRX-2843 may be particularly well-suited for treatment of ETP-ALL. The Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signaling pathway is also hyperactivated in ETP-ALL relative to typical T-ALL and inhibition of this pathway is efficacious in xenograft models of ETP-ALL (Maude et al Blood 2015).
Methods
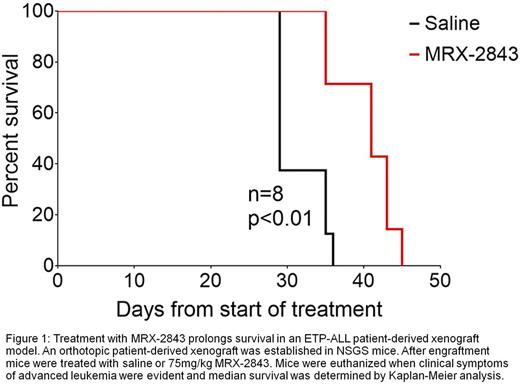
Publicly available mRNA expression data were used to assess MERTK expression in T-ALL patient samples. MERTK protein expression was determined in ETP-ALL cell lines and patient samples by immunoblot. ETP-ALL cell lines were cultured with vehicle or MRX-2843 and expression of phosphorylated and total MERTK and STAT5 were determined by immunoprecipitation and/or immunoblot. Alternatively, cells were stained with PoPro-1-iodide (POPRO) and propidium iodide (PI) dyes and apoptotic (POPRO+, PIneg) and dead (PI+) cells were analyzed by flow cytometry. Orthotopic xenografts were established in NSGS mice using an ETP-ALL patient sample. Leukemia burden in peripheral blood was monitored by immunophenotyping with an anti-human CD45 antibody. After engraftment (1.86 +/- 0.43% peripheral blasts), mice were treated once daily with 75 mg/kg MRX-2843 or saline vehicle administered by oral gavage. Mice were euthanized when clinical symptoms of advanced leukemia were evident and median survival was determined by Kaplan-Meier analysis.
Results
MERTK mRNA was expressed at significantly higher levels in ETP-ALL cell lines and patient samples relative to other T-ALLs. MERTK protein was expressed in all of the ETP-ALL cell lines (n=2), ETP-ALL patient samples (n=2), and near-ETP-ALL patient samples (n=1). In contrast, only 60% of T-ALL cell lines (n=5) expressed MERTK. Of note, the Loucy ETP-ALL cell line expressed the highest levels of MERTK mRNA and protein. Treatment with MRX-2843 decreased phosphorylated MERTK and downstream STAT5 signaling in the ETP-ALL cell line PEER in a dose-dependent manner. Treatment with MRX-2843 also induced dose-dependent cell death in the ETP-ALL cell lines PEER (43.2% versus 16% in vehicle-treated cultures, p<0.01) and Loucy (36.6% versus 19.2% in vehicle-treated cultures, p<0.05). Moreover, in an orthotopic patient-derived xenograft model of ETP-ALL, treatment with MRX-2843 for 29 days reduced peripheral blood disease burden by 53.9% (p<0.001) and decreased spleen weight by 64% (p<0.001) compared to vehicle-treated controls. Furthermore, mice treated with MRX-2843 survived for a median of 41 days post-treatment compared to 29 days for control mice (Figure 1, n=8/group, p=0.003).
Conclusions
MERTK is preferentially expressed in ETP-ALL relative to typical T-ALL. MRX-2843, a dual MERTK/FLT3 inhibitor, has robust therapeutic activity in cell culture and xenograft models of ETP-ALL and the mechanism of activity may be, in part, mediated by STAT5 signaling. These data validate MRX-2843 as a novel agent with potential for clinical application in patients with ETP-ALL. In particular, combination therapy targeting MERTK and the JAK/STAT pathway may be an attractive therapeutic option for patients with ETP-ALL.
Frye: Meryx: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; University of North Carolina: Patents & Royalties. Wang: Meryx: Equity Ownership; University of North Carolina: Patents & Royalties. Earp: Meryx: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. DeRyckere: Meryx: Equity Ownership. Graham: Meryx: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal